A SHORT HISTORY OF THE DEVELOPMENT OF DIGITAL MAMMOGRAPHY
INTRODUCTIONa
I apologize if I have left out individuals who were instrumental in the development of digital mammography. As with many advances, there are almost certainly those who make important contributions, but may not receive the recognition that they deserve. I will do my best to provide the highlights, but I am certain it is far from a complete story.
A SHORT HISTORY OF MAMMOGRAPHY
There are two very good reviews of the history of Mammography. One by Bassett and Gold in the journal Radiology () and the other by Joe and Sickles ().
X-ray imaging of the breast was undertaken not long after Roentgen first discovered “x-rays”. In the early years of the 1900’s Salomon in Germany x-rayed several 1000 mastectomies. Stafford Warren in the U.S. categorized the appearance of normal breasts on mammograms. Lockwood and Gershon-Cohen also contributed to understanding the x-ray images of the breasts. Leborgne performed mammograms in the 1950’s and, apparently, was the first to recognize the relationship of microcalcifications to cancers. Finally, in 1960, Egan described a technical approach to mammography that standardized the technique and provided the opportunity for mammography to be performed in a systematic and reproducible fashion ().
In the 1960’s Phillip Strax, a radiologist, working with Sam Shapiro, a biostatistician, took the Egan method and ran the first Randomized, Controlled Trial of mammography screening within the Health Insurance Plan of New York (HIP) and, using plane “industrial” film, were able to show that early detection could save lives.
Plain x-ray film required high doses to make the images, and the contrast was not very good. At that time there was no compression of the breast so that the thinner front of the breast was often over exposed and “burned out” so that not much could be seen, while the thicker back of the breast was often underexposed and compromised by scatter x-rays. The images were quite poor.
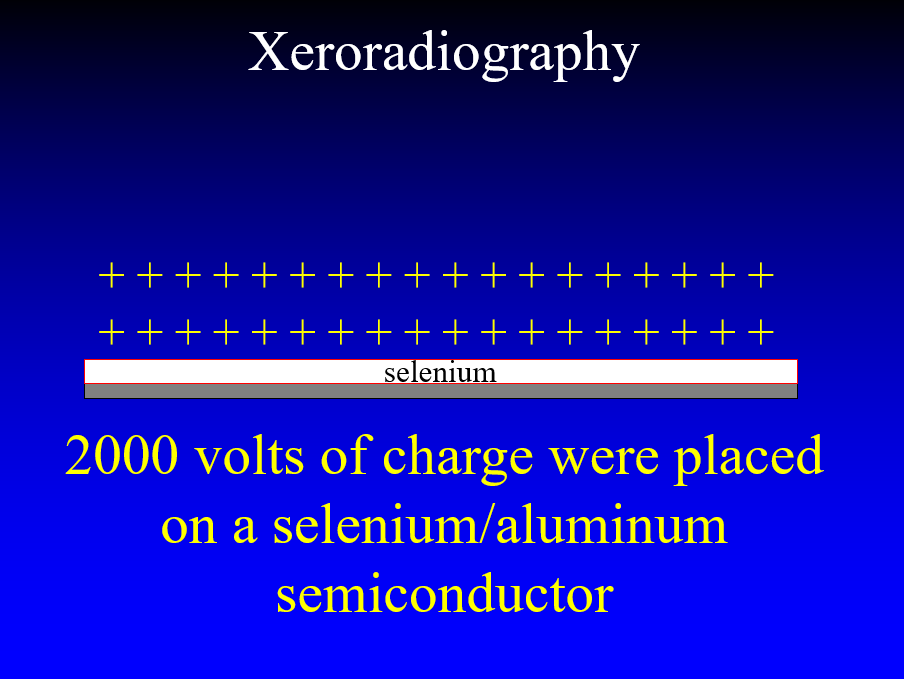
In the 1970’s John Wolfe worked with the Xerox company to take the technology that they had created for making copies of documents and used it to perform x-ray imaging of soft tissues. The technique employed an aluminum plate coated with selenium. With the plate placed in an insulating box a high voltage could be placed on the surface of the plate. X-rays passing through the breast discharged the plate in proportion to the x-rays reaching each point on the plate leaving a charge image.
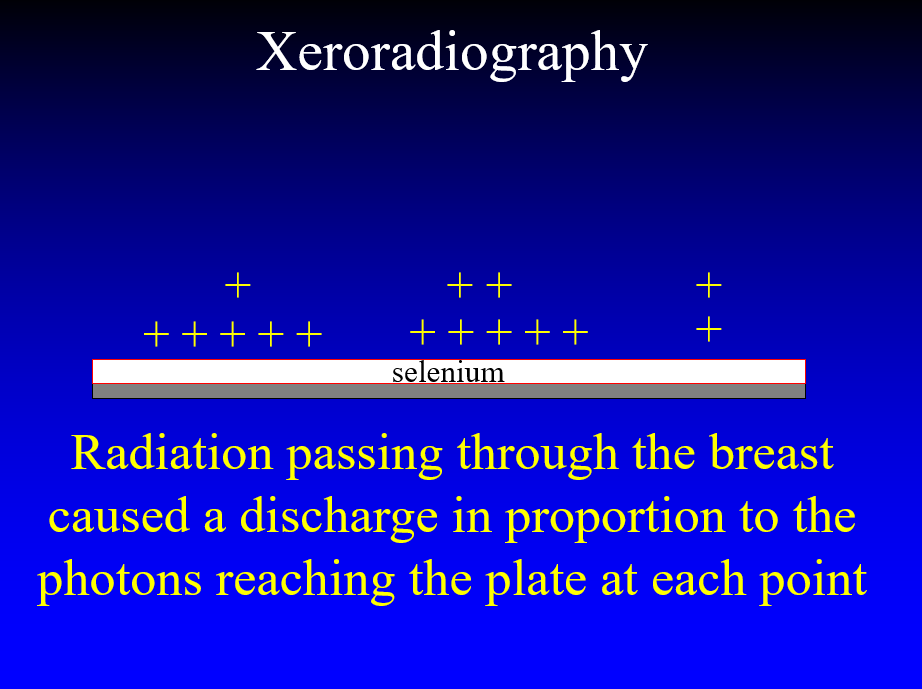
The plate was removed from the box and toner particles were applied to the plate and the electrostatic charges caused the particles to adhere to the plate in proportion to the charge that the x-rays had left on its surface.
A plastic-coated sheet of cardboard was applied and the toner was melted into its surface forming the “Xerogram”. This basic principle is fundamental to present day full field digital detectors, but, instead of toner to show the charge distribution, the residual charge is read electronically, directly from the plate.
Although a far superior image to plain film, the Xerogram still required fairly high doses of radiation, and Bailar’s concerns in the 1970’s () provided the impetus to reduce dose. This led to the development of screens coupled with single emulsion film that allowed for shorter exposures with lower dose and high resolution. Dedicated mammography machines using rigid compression to flatten and form the breast into a rectangular solid with a more uniform thickness to improve image quality allowed the screen/film combinations to replace Xeroradiography. Grids were added to reduce scatter, and, in the 1980’s, Screen/Film mammography in dedicated mammography systems became the dominant method for screening with a lower dose than previously possible using film alone.
THE COMPUTER AGE
As computers were being used more and more in medicine, efforts were made to apply them to mammography. In the 1980’s we digitized screen/film images and were able to transmit the images electronically. Between 1993 and 1997 my Research Director, Richard Moore, showed that we could send digitized mammographic images out to a satellite in geosynchronous orbit in space and back, with no loss of information. It took, on average, 2.2 minutes to send and receive a single image.
Screen/film mammograms were digitized so that computers could be used to try to assist radiologists in detecting cancers. There was great interest, but ultimately disappointing results from “Computer Aided Detection” (CAD). In CAD we (radiologists) provided cases that were used to “teach” computers what we were looking for on mammograms. Ultimately CAD was not much help.
In the 1980’s direct digital x-ray imaging was under development for various parts of the body. There were numerous efforts to collect the images in computers. One very ambitious company was American Science and Engineering (AS&E). They built a prototype “flying spot” that used a very large wheel with varying collimators that took an x-ray beam and collimated it into a spot and, as the wheel spun, a “spot” of x-rays moved in raster fashion across the chest of a patient with a single large detector collecting the x-ray transmission at each point. This was very efficient since the detector collected the data at each point, separately, but it took a relatively long time for each study and lacked sufficiently high resolution.
Different detector materials were devised. It was found that there were some phosphors that could be “stimulated” whose energy level could be elevated when hit by x-rays coming through a body part, and then the energy could be measured and recorded at each point by scanning the detector with a laser.
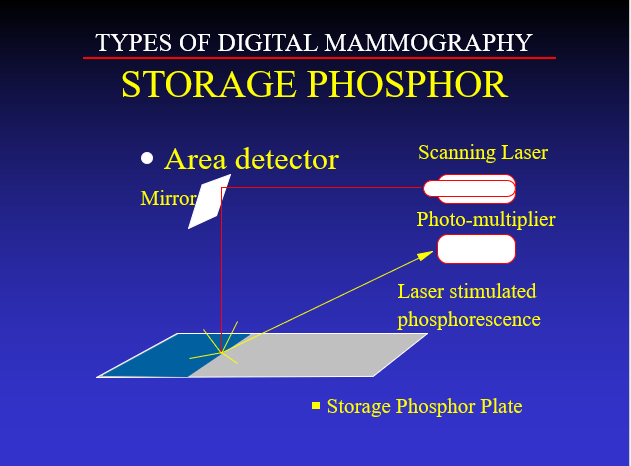
This approach proved to be insufficient for imaging the breast ().
In the early 1980’s AS&E, led by Martin Annis PhD. developed an extremely efficient detector designed by Paul Bjorkholm PhD. that was used to create a prototype line scanning system. The x-ray photons were collimated by a pre-breast slit and scatter was blocked by a post breast slit. The x-ray photons passing through the slits and the bresat entered the edge of a fluorescent screen tipped to be oriented parallel to the x-ray beam. A photodiode array was along the x-ray path so that there was a long path for the photons to be stopped and to generate light, but a short path to the photodiodes, greatly reducing light spread in the detector and generating a sharp image. The device was very high resolution and had almost complete scatter rejection.
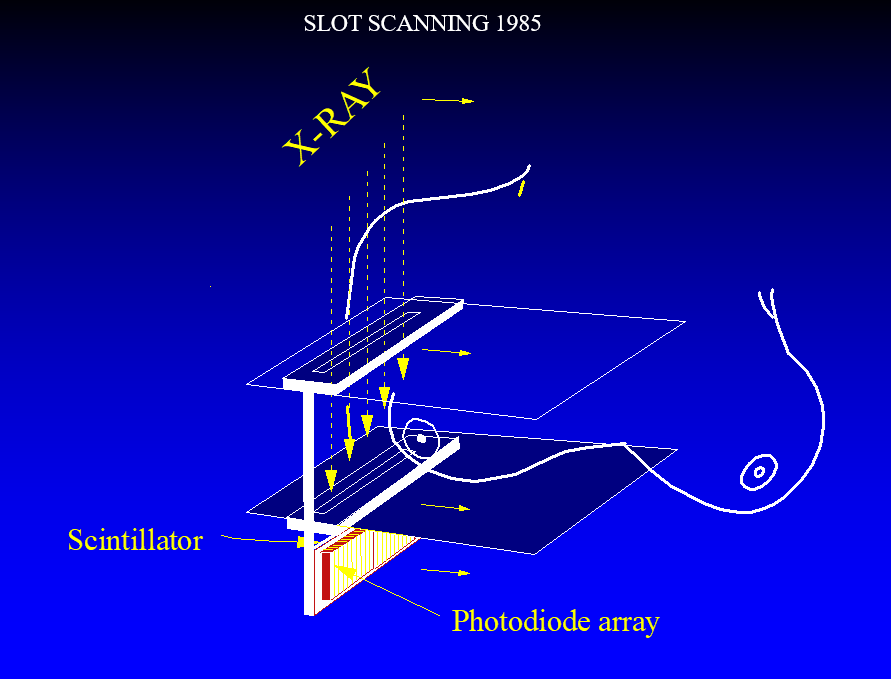
A prototype was installed at the Massachusetts General Hospital, and we obtained the first high resolution digital mammograms of volunteers. Unfortunately, the system was too far ahead of its time and AS&E could not sustain the effort and terminated its development.
X-ray systems for imaging other parts of the body were under development in the late 1980’s. Our Food and Drug Administration (FDA) only required a “510K” process to get these systems approved. Essentially a 510K required that, for example, you obtain a chest x-ray using conventional screen/film imaging and compare it to a second image obtained with the new digital chest x-ray device. It literally required a handful of comparisons on which the screen/film image and the digital image appeared sufficiently similar, that the FDA would approve the new digital system.
Unfortunately, the FDA approval was markedly delayed for Full Field Digital Mammography (FFDM) because an administrator, who was not a physician, had moved from the National Cancer Institute, where he had been opposed to mammography screening, to a position at the FDA, and had convinced the FDA that FFDM would be so new that radiologists would not be able to interpret the studies and would require many more women to return for additional evaluation. He was not a radiologist, and this was simply nonsense, but, as a result, and given the very high public interest in mammography screening, the FDA required that FFDM would have to go through the very detailed, complex, and expensive process usually reserved for completely new systems that is known as a “Premarket Approval” or “PMA”. Few companies had the resources or interest to undergo this approach and the development of FFDM systems was greatly delayed as a result.
In 1992 Etta Pisano, M.D. organized the Digital Mammogrpahy Development Group that would become the National Digital Mammography Development Group and ultimately the International Digital Mammography Development Group initially consisting of my group at the Massachusetts General Hospital that included Richard Moore and Loren Niklason, PhD., a physicist who had just joined us. Stephen Feig M.D. at Thomas Jefferson Medical Center in Philadelphia, Martin Yaffe, PhD and Donald Plewes PhD both from Stonybrook in Canada. Our task was to help General Electric (GE) and Fisher Imaging develop their prototypical Full Field Digital Mammography systems (FFDM).
AN OVERVIEW OF DIGITAL DETECTORS FOR MAMMOGRAPHY
A good overview of the various detectors that have been developed for mammography can be found in the paper by Yaffe and Mainprize ()
Fisher Imaging was building a line scanning system which used a collimated line of x-rays that was mechanically scanned across the breast with the detector aligned with this beam with scatter reduced by pre and post breast slots. The detector consisted of a phosphor detector array in which the x-rays were converted to electrical signals. This was similar to the AS&E system, but the photodiode array was in line with the x-ray beam. The array and collimated beam were scanned across the breast. Ultimately, this system was simply too complex and did not achieve commercial success.
General Electric had developed a flat panel detector that consisted of Cesium Iodide crystals grown epitaxially in columns on a photodiode array. The Cesium Iodide converts the x-ray photons to light photons which are converted by the photodiodes to electrical signals in the array. Their initial system was a digital detector for angiography that they were hoping to scale up for mammography.
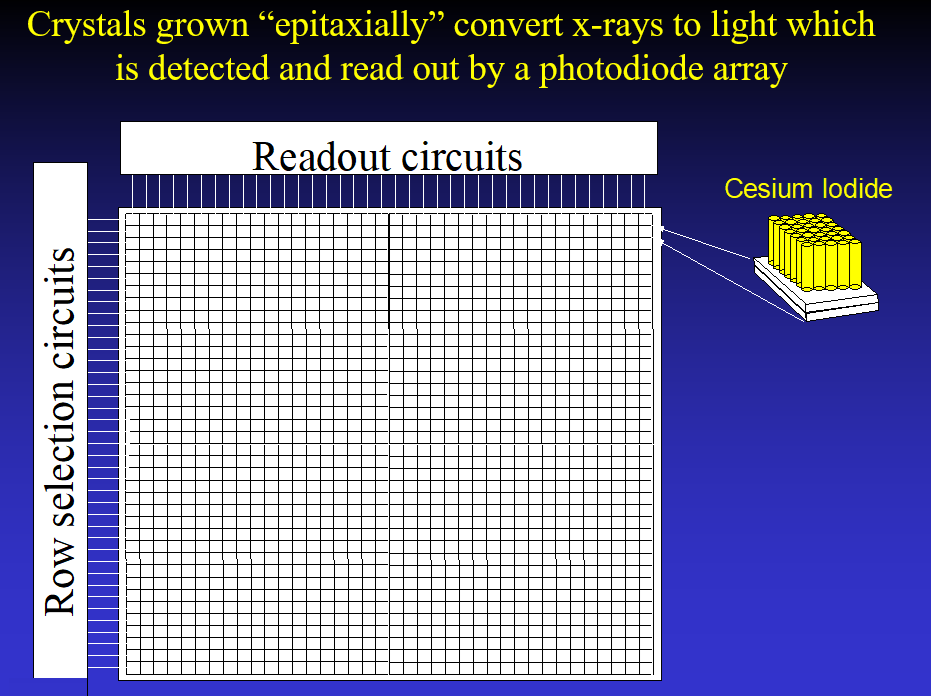
Having read about the concept of tomosynthesis in the 1970’s and realizing that it might have major advantages for mammography (), I, nevertheless, had to wait until the 1990’s to obtain a prototype digital mammography system. To develop “Digital Breast Tomosynthesis (DBT)” we needed a high-resolution system that could be read out quickly enough to allow us to take multiple images over a short period of time. The GE detector had these characteristics, so we partnered with GE within the DMDG to help them develop their FFDM and get access to their detector.
One of the early FFDM systems was based on a detector system developed by Bennett and Northrop Grumman manufacturing that was based on a system to be marketed for “spot” imaging since the field of view of the detector was small. In 1994 Bennett obtained FDA approval for a phosphor coating on the ends of numerous glass fibers that tapered from an approximately 5 sq cm area down to the charged coupled device that converted the light photons to electrical signal.
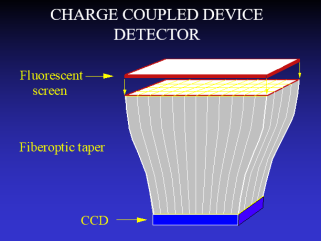
LORAD was a company that developed a similar CCD camera. Trex was unable to get FDA approval for a tiled system that joined multiples of these CCD cameras together, but Hologic succeeded and combined the small Field of View CCD cameras into a larger detector by fusing 12 cameras (4x3 array) into a wide field of view. This device was short lived as Hologic changed to the detector design using selenium, that is still used by most companies, with the exception of GE that still uses its Cesium Iodide detector.
Most companies now use a detector that is based on the Xeroradiography system of the 1970’s and early 1980’s. Amorphous Selenium is fused to metal a plate forming a semiconductor. A homogenous charge is placed over the detector surface, and x-rays passing through the breast discharge the plate in proportion to the amount of radiation striking the plate at any point. Instead of blowing toner onto the plate to reveal the latent image, as with Xeroradiography, the charge is now read directly from the plate.
FDA WANTED A POST APPROVAL SCREENING STUDY
The FDA was also going to impede the introduction of FFDM by not only requiring a PMA, but they were also going to require the companies that were given approval, to perform a huge, post approval screening trial. This is not uncommon for companies developing therapeutic approaches to cancers, but it was unheard of for imaging. Morgan Nields, who was Head of Fisher Imaging, reportedly went to Congress to explain that his small company could not afford a large post approval trial and Congress earmarked $25 million to support these trials. In 2000 GE applied to the FDA with their PMA for Digital Mammography. Independently, I went to a meeting of the advisory committee to the FDA that was reviewing GE’s submission. I testified that the FDA should have never required a PMA for FFDM since it was obvious that it was equivalent to Screen/Film (S/F) mammography, and I showed a breast cancer on FFDM and S/F mammography and explained to the committee that I could not see a difference, and that they could not see a difference. We understood the physics, and because FFDM and S/F mammography images were indistinguishable FFDM should be approved. I explained that the FDA should have not required a PMA, and that, certainly, a post approval screening trial was not necessary (). The Committee agreed with me and unanimously supported GE’s PMA and advised the FDA to drop the requirement for a post approval screening trial. The FDA agreed and dropped the trial requirement and approved GE’s PMA.
Unfortunately, I suspect that the $25 million that was earmarked by Congress and no longer needed, was used instead, and wasted on the DMIST trial () which was, essentially, the Post Approval trial that the FDA had dropped. Although it has been claimed that DMIST proved that FFDM was superior to SF in some women, the overall study showed that SF and FFDM were comparable. The true advantages for FFDM are the logistics which changed dramatically. Chemicals and film processors were no longer needed. Film libraries were no longer needed. Film management was no longer needed. Images would never be lost and could be viewed by unlimited viewers simultaneously anywhere in the World. Perhaps of greatest significance is the fact that FFDM opened the technological doors for the development of Digital Breast Tomosynthesis (DBT) which has dramatically increased the detection of curable breast cancers. Although computer aided detection (CAD) has not lived up to its original promise, the next advance using “Artificial Intelligence” (AI) will almost certainly become a major improvement in the early detection of breast cancer. FFDM has also allowed for the use of Dual Energy Subtraction, so called “Spectral Mammography”, that is now being used to detect the neovascularity of breast cancers ().
There has been a steady evolution of x-ray mammography from plain film to Xeroradiography, to Screen/Film systems and then Digital Mammography. They have been incremental improvements until the development of DBT which has made a major improvement in the detection of invasive cancers at a small and potentially curable stage. Other advances are likely now that computerized mammography has replaced S/F mammography such as introducing ultrasound into the DBT systems to provide DBT/US fusion images to further improve our ability to detect breast cancers at a curable stage in their growth. Dual energy could also be used to improve our detection of the clustered microcalcifications that indicate Ductal Carcinoma in Situ (DCIS) and are sometimes the only indication of an associated invasive breast cancer. Dual energy following the injection of iodinated contrast material to demonstrate the neovascularity of cancers will also enhance the detection of cancers at an earlier and potentially curable stage.
We have dropped the death rate for breast cancer by almost 50% since 1990, in large part because of early detection. Having digital images that that can be analyzed by computers using AI will allow us to bring computers to bear on early detection and should improve our ability to, efficiently, detect more cancers at a time when cure is possible and further reduce deaths from breast cancer.
Bassett LW, Gold RH. The evolution of mammography. AJR Am J Roentgenol. 1988 Mar;150(3):493-8. doi: 10.2214/ajr.150.3.493. PMID: 3277343.
Joe BN, Sickles EA. The evolution of breast imaging: past to present. Radiology. 2014 Nov;273(2 Suppl):S23-44. doi: 10.1148/radiol.14141233. PMID: 25340437.
Egan RL. Experience with mammography in a tumor institution. Evaluation of 1,000 studies. Radiology. 1960 Dec;75:894-900. doi: 10.1148/75.6.894. PMID: 13725888.
Bailar, JC. Mammography: A contrary view. Ann Intern Med 1976 84:77-84.
Oestmann JW, Kopans DB, Hall DA, McCarthy KA, Rubens, JR. A comparison of Digitized Storage Phosphors and Conventional Mammography in the Detection of Malignant Microcalcifications. Invest. Rad. Vol. 23 No. 10 1988; 725-728.
Yaffe M, Mainprize J. Detectors for Digital Mammography Technology in cancer research & treatment, 2004-08, Vol.3 (4), p.309-324
Kopans DB. Digital breast tomosynthesis from concept to clinical care. AJR Am
J Roentgenol. 2014 Feb;202(2):299-308.
https://essaydocs.org/radiological-devices-panel-meeting.html
Kopans DB. A history of DMIST and its implications - Limited resources should be better spent. Clin Imaging. 2021 Jun 17:S0899-7071(21)00251-5. doi: 10.1016/j.clinimag.2021.06.005. Epub ahead of print. PMID: 34172355.
Lewin JM, Isaacs PK, Vance V, Larke FJ. Dual-energy contrast-enhanced digital subtraction mammography: feasibility. Radiology. 2003;229:261-8.


