PREOPERATIVE MAMMOGRAPHICALLY GUIDED LOCALZATION (1)
INTRODUCTION
Screening and the earlier detection of breast cancer, that began in the mid 1980’s in the U.S., is the main reason that deaths from breast cancer, unchanged for 50 years, began to fall in 1990¹. The death rate has continued to decline as we have improved our ability to detect breast cancers earlier. Tabar has shown that if cancers are detected and treated when 1 cm or smaller, the 10 year survival is over 90%². Consequently, screening requires an ability to aggressively diagnose small cancers.
Compounding the problem of early detection is the fact that there are many benign lesions (cysts, fibroadenomas, and others) that are detected by screening that cannot be differentiated from malignant lesions without some form of histological or pathological analysis. The yield of cancers on image guided needle biopsies of mammographically detected lesions in the U.S. is 20%-40%. In the U.S. image guided core needle biopsy, that provides histological information, has become the standard of care to determine the importance of a screen detected lesion. These needle biopsies can be guided by Full Field Digital Mammography, Ultrasound, Digital Breast Tomosynthesis, Magnetic Resonance Imaging and Spectral Mammography.
Ultimately, if surgical biopsy is the main method for obtaining histological diagnosis, or if the core needle biopsy result is indeterminate, or suggestive of malignancy, surgical excision remains the main intervention. This is also the case when the diagnosis of cancer has been made and the lesion needs to be surgically removed. Consequently, we need to be able to guide surgeons to areas of concern so that they can safely and accurately remove what needs to be removed while not sacrificing breast tissue, unnecessarily.
HISTORY
In the early years of screening, it took a while for surgeons to accept the fact that mammography was finding breast cancers that were so small that they could not be palpated even when the location of the lesion was known. Prior to the development of image directed guide placement, there was a period when surgeons would remove large amounts of breast tissue based on their estimate of the expected location of a lesion. In my own experience, when a senior surgeon removed the upper out quadrant of a patient’s breast and missed what ultimately proved to be a cancer that was only visible on mammography, it became clear that we needed to guide surgeons to the area of concern. We also recognized that since 20%-40% of the lesions that raised concern and needed to be biopsied, proved to be malignant (60%-80% are not cancer), the surgeon needed safe and accurate guidance to accurately remove a lesion while minimizing any cosmetic damage to the breast. In the beginning these biopsies were performed under general anesthesia in the main operating rooms. Since this was expensive and placed patients at unnecessary risk, we realized that, if we could accurately guide surgeons, the procedure could be done in an outpatient operating room using local anesthesia with much greater safety and efficiency.
EARLY “NEEDLE LOCALIZATIONS”
The early “localizations” were performed using plain hypodermic needles that were placed close to the suspicious lesion based on the mammogram. These were problematic since the needles were placed “free hand” and were not always close to the lesion. In addition, they could fall out with the motion of the breast when the patient was positioned for surgery or when the breast was being prepared with sterile solutions prior to the operation. Ultimately, I invented a wire guide that, once the needle was accurately placed, the guide could be passed through the needle. The end of the wire was bent in such a way that allowed it to spring open and hook into the tissue³. The wire can be placed under imaging guidance prior to surgery and the guide positioned so that the surgeon can follow the wire to the volume of tissue that needs to be removed. I also developed techniques for positioning the wire guide that were safe and very accurate⁴′⁵.
Accurate mammographically guided needle positioning requires a fenestrated compression paddle.
For mammographically guided needle localization the breast is held in compression using a paddle that has a window in it with markings along the edges of the window.
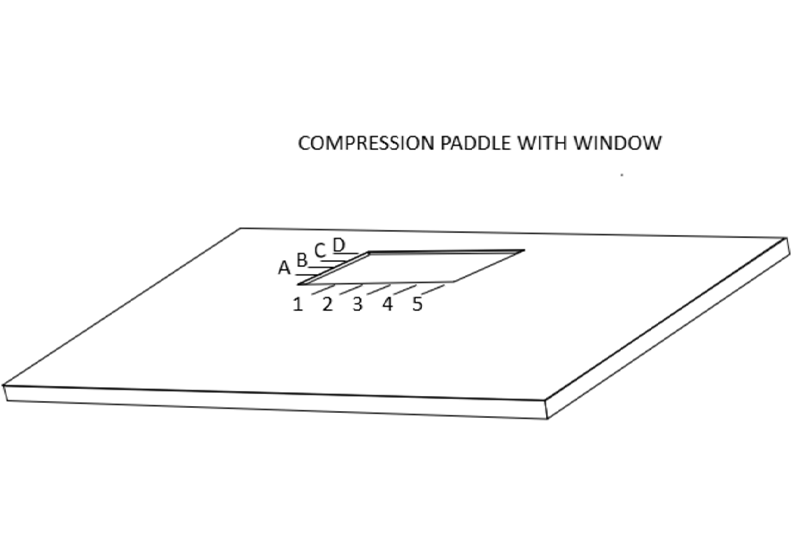
This allows the radiologist to determine the x and y coordinates of the lesion when the breast is positioned so that the lesion is visible on the mammogram “in the window”.
ALIGNING THE CENTERING LIGHT
In order to facilitate the accurate positioning of needles in the breast, the centering light in the mammography gantry needs to be aligned with the x-ray beam. This can be checked by placing a sponge in the compression system with the window compression in place so that needles can be placed in the corners of the window.
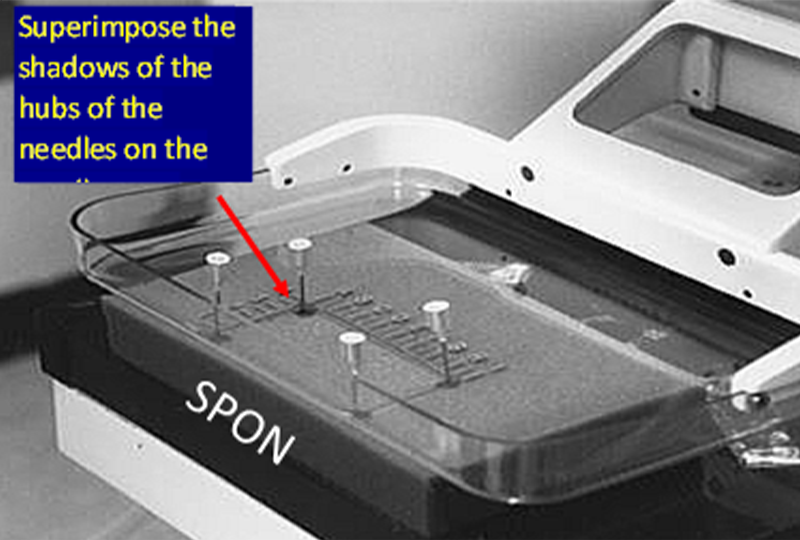
The centering light is turned on and each needle is positioned so that the shadow of the hub of the needle is centered on the shadow of the needle where is penetrates the sponge, and then the needle is advanced into the sponge. If done correctly you will see that the needles are tilted and not perpendicular to the paddle or detector. This is because, like the x-ray beam, the centering light is divergent, it angles out from the center of the chest wall side of the paddle and detector (where it is almost perpendicular).
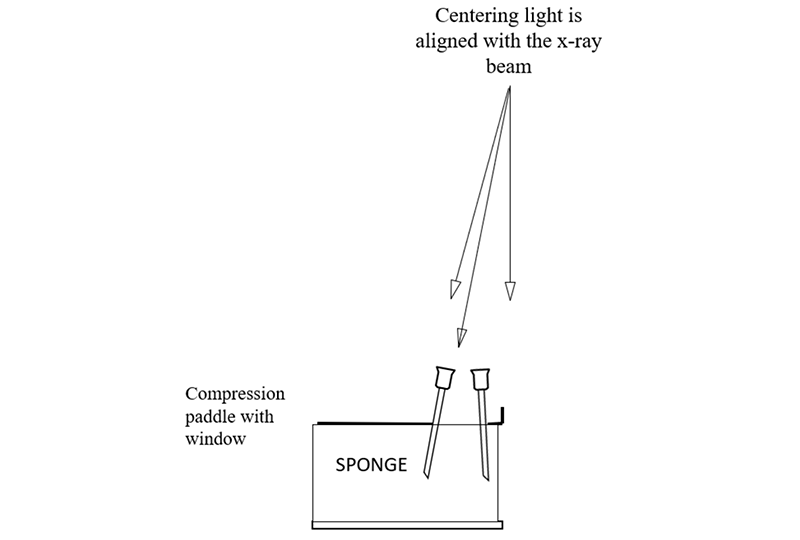
When the needles are aligned, an x-ray image is then taken. If the shadows of the needle hubs are not centered on the needle shaft in the images, then this means that the centering light is out of alignment and this needs to be corrected. Once the centering light is aligned with the x-ray beam then it can be used like a fluoroscope to position the localization needle accurately through or alongside the targeted lesion.
POSITIONING THE PATIENT
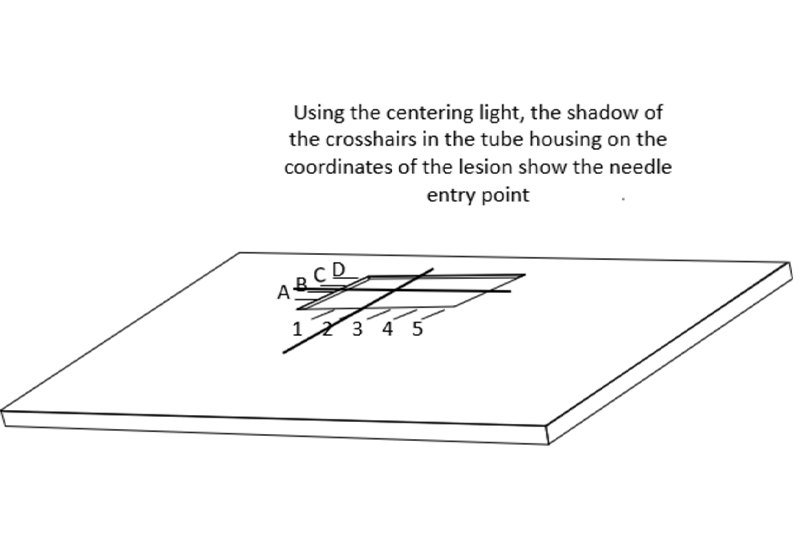
Once a lesion has been identified and the decision is made to perform a surgical excision, the three-dimensional location of the lesion in the breast needs to be determined. The radiologist then should pick the shortest distance to the lesion from the skin when the needle is passed parallel to the chest wall. This latter requirement is important so that it is virtually impossible to, inadvertently, puncture the chest wall, or worse into the lung or the mediastinum.
When the shortest distance has been determined, I select a needle wire combination that is longer than the distance that I am expecting from the skin to the lesion. It is critical to be certain that the needle and wire are “too long” so that they can pass beyond the lesion during the procedure to be certain that the hook is just beyond the lesion with the lesion on the thickened segment of the wire guide.
The patient is then positioned in the best mammographic position to place the skin entry site in the window of the compression paddle. There is no need to adhere to specific mammographic projections since the goal is to place the guide in or alongside the lesion using the shortest distance to the lesion so that the surgeon, following the wire to the lesion does not have a long dissection.
The patient should be seated and as comfortable as possible. The only major problem I have ever had is when a patient has a vasovagal reaction. The team should be prepared for this. Ideally the chair can be tilted into the Trendelenburg position should the patient become faint. It is best if the technologist can distract the patient during the procedure with unrelated talk. It is important to not allow the patient to see the needle since this can result in a vasovagal episode. I turn the patient’s head so that she cannot see the needle before I introduce it into her breast while jokingly stating “now you have a lovely face, but I need to move it out of my pictures”. The patient’s breast needs to remain stationary throughout the procedure, but every effort should be made to distract her and not raise her concern.
REFERENCES
1.Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019 May 1;125(9):1482-1488.
2.Tabàr L, Fagerberg G, Duffy SW, Day NE, Gad A, Gröntoft O. Update of the Swedish two-county program of mammographic screening for breast cancer. Radiol Clin North Am. 1992 Jan;30(1):187-210. PMID: 1732926.
3.Kopans DB, Deluca S. A modified needle-hookwire technique to simplify the preoperative localization of occult breast lesions. Radiology 1980; 134:781.
4.Kopans DB, & Meyer JE, The Versatile Spring-Hookwire Breast Lesion Localizer. AJR 1982;138: 586-587
5.Kopans DB, Meyer JE, Lindfors KK, & McCarthy KA, Spring-Hookwire Breast Lesion Localizer: Use with Rigid Compression Mammographic Systems. Rad 1985;157: 505-507


