超声引导下的空芯针穿刺
从筛查到诊断
乳腺X射线摄影筛查是乳腺癌防治的主要进展之一。通过早期发现,美国自1990年以来的乳腺癌死亡率下降40%以上。当然,筛查并不能检查出所有的癌症,也不能检查出所有还能被治愈的癌症。此外,筛查可以发现乳腺中的良性结构,这些良性结构不是癌症,但可能无法用影像学方法将其与乳腺癌区分开来。在美国,参与筛查的女性中大约有10%会被召回,进行额外的乳腺X射线摄影或超声检查。这些女性中的大多数人会被告知:额外的影像诊断没有显示出什么问题,她们只要继续每年的筛查。一部分女性可能会被建议无需立即处理,但她们应该在6个月后再来检查,以确定在她们的乳腺X射线摄影上的发现是稳定的、良性的。小部分女性(约占筛查女性的2%)会被要求进行影像引导下的穿刺活检,以确定筛查中发现的病变组织性质。相比细针穿刺活检(FNA),我更喜欢空芯针活检,因为空芯针的组织学比细胞学提供更完整的诊断分析。
超声是区分囊肿和实性肿块的最好方法
当通过筛查发现了不确定的病变,并通过额外乳腺X射线摄影(如果需要)确认时,接下来几乎都会被要求再做超声检查。除非在乳腺X射线摄影中发现环形结构内有形态随体位变化的可明确为“牛奶样”钙化,否则仅乳腺X射线摄影是无法区分单纯良性囊肿和实性肿块的。
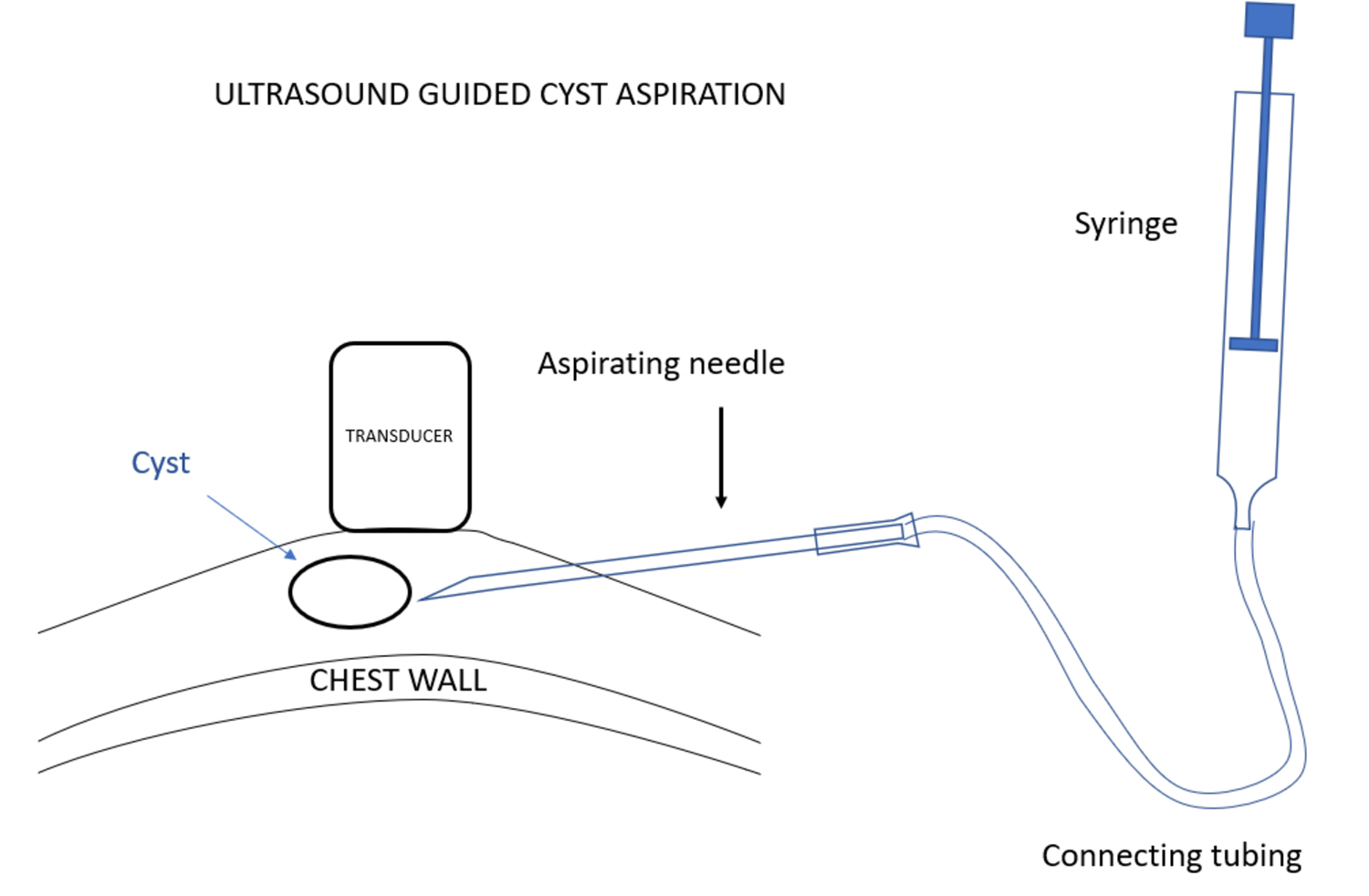
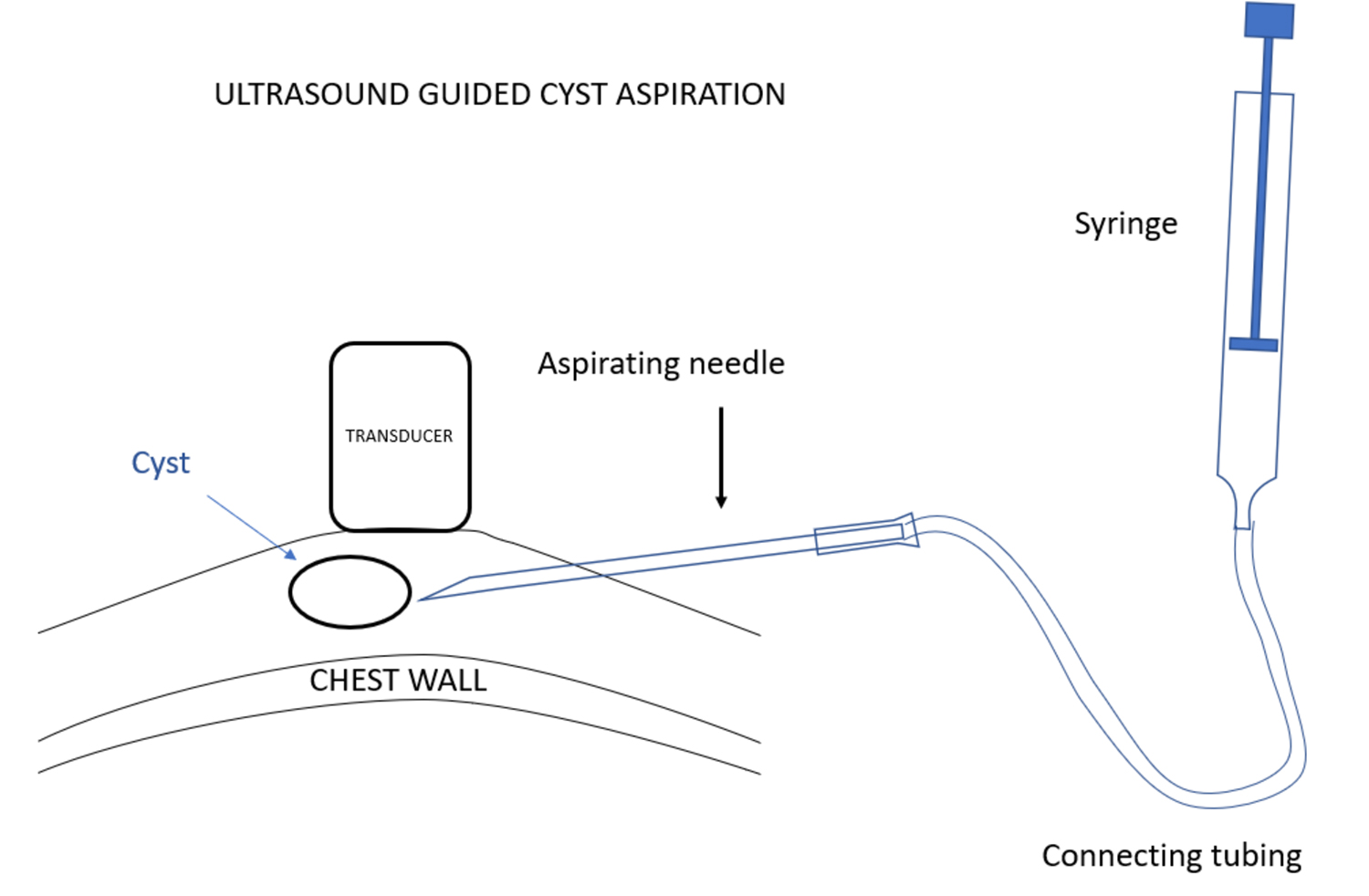
使用超声波可以很容易确诊乳腺X射线摄影无法确认的囊肿。单纯性囊肿是良性的,除非引起疼痛,囊肿引起疼痛的情况是相对少见的,否则无需进一步干预。在这种情况下,可以通过影像学引导进行囊肿穿刺。影像学引导下的囊肿抽吸可以使用与活检相同的技术进行。不过,囊肿抽吸是在超声引导下使用抽吸针而不是通过引导器插入活检设备。针头通过连接导管与注射器连接,放射科医生在超声观察下插入针头,当针头穿进病变时,助手可使用注射器进行抽吸。
超声引导下定位
无论是引导下的囊肿穿刺,还是对实性肿块进行活检,超声引导下的定位术都是最安全、最简单、最准确的方法之一。患者取仰卧位,将同侧手臂置于头后,使乳房在胸壁上变平。也可以调整患者体位以便更好地做乳房定位。我们的目的是定位病人的乳房和病变,以便为活检针提供最安全的进针路径。安全是最重要的。针头的位置应尽可能与胸壁平行,以便在取样时针尖可以穿进病变而不触及胸壁。
对患者乳腺进行超声扫查以确定病变的位置,并在病变正上方的皮肤进行标记。然后,将超声探头放置在病灶位置,标记皮肤穿刺点。选择皮肤穿刺点是为了当针头进入皮肤向病变方向前进时,始终可以在探头下看到针尖。
超声探头上套一个含有耦合剂的无菌套。然后用碘伏和酒精进行穿刺点皮肤的消毒,并将皮肤穿刺点置于开口的无菌布上,对穿刺点进行局部麻醉。
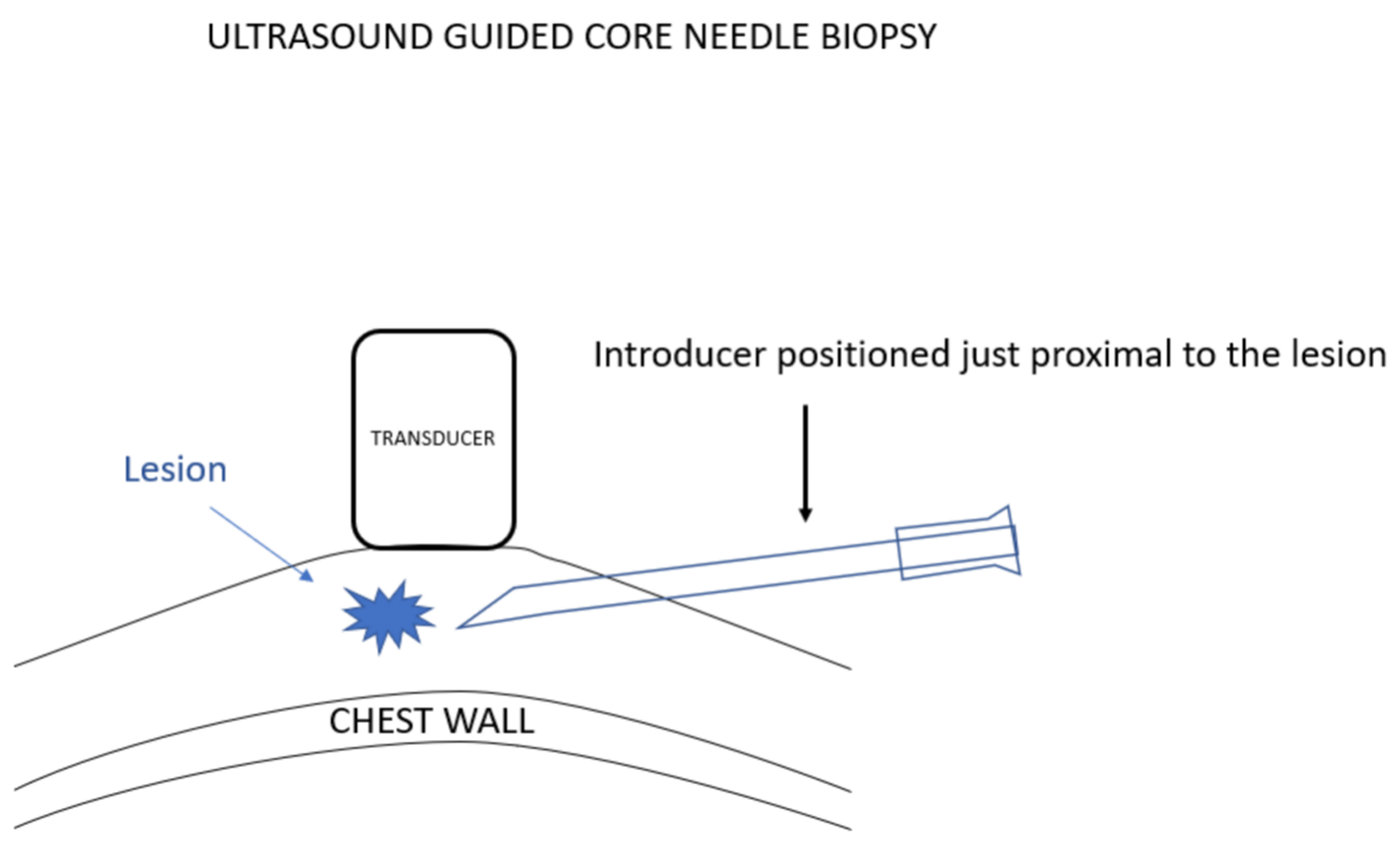
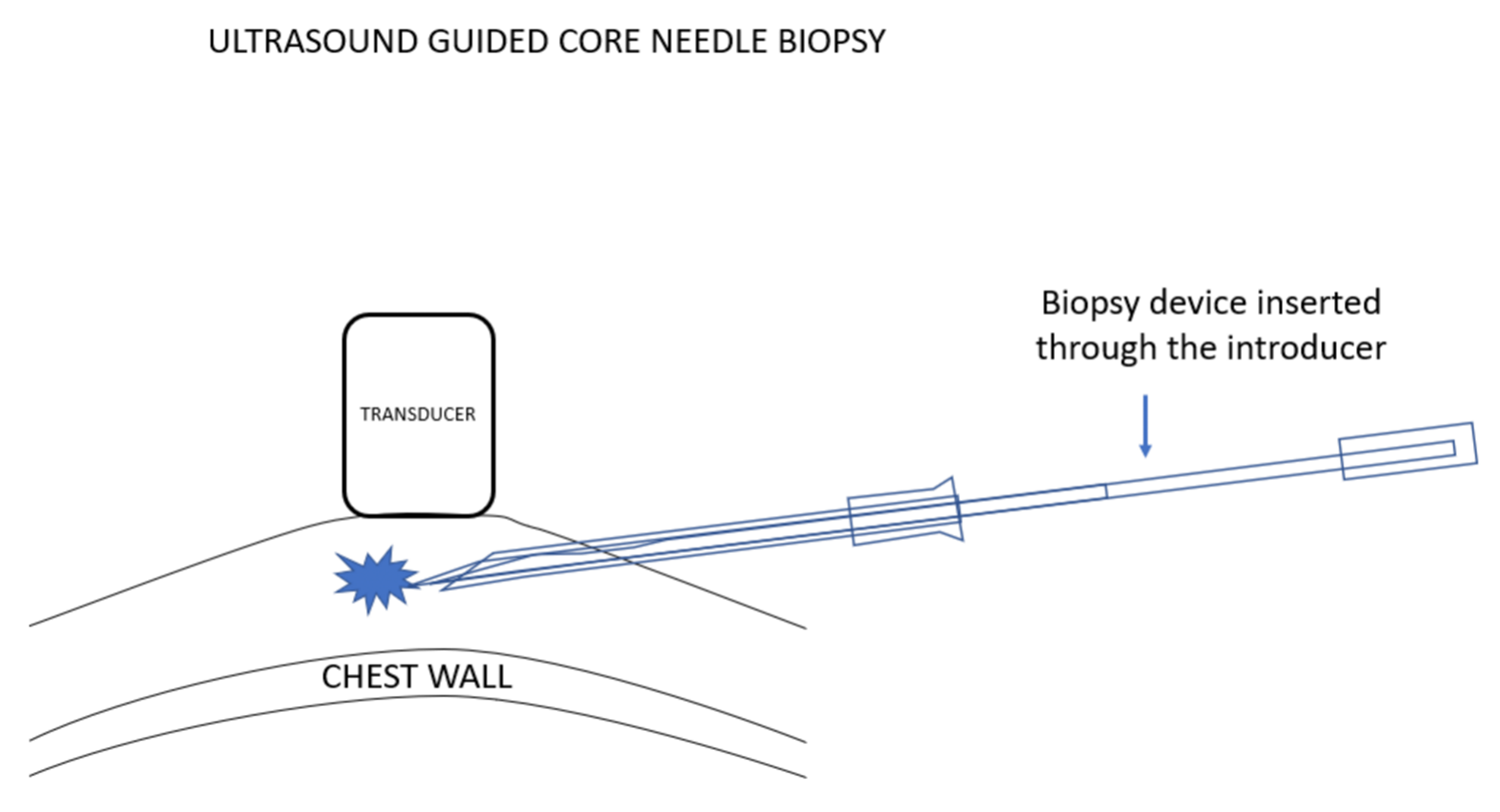
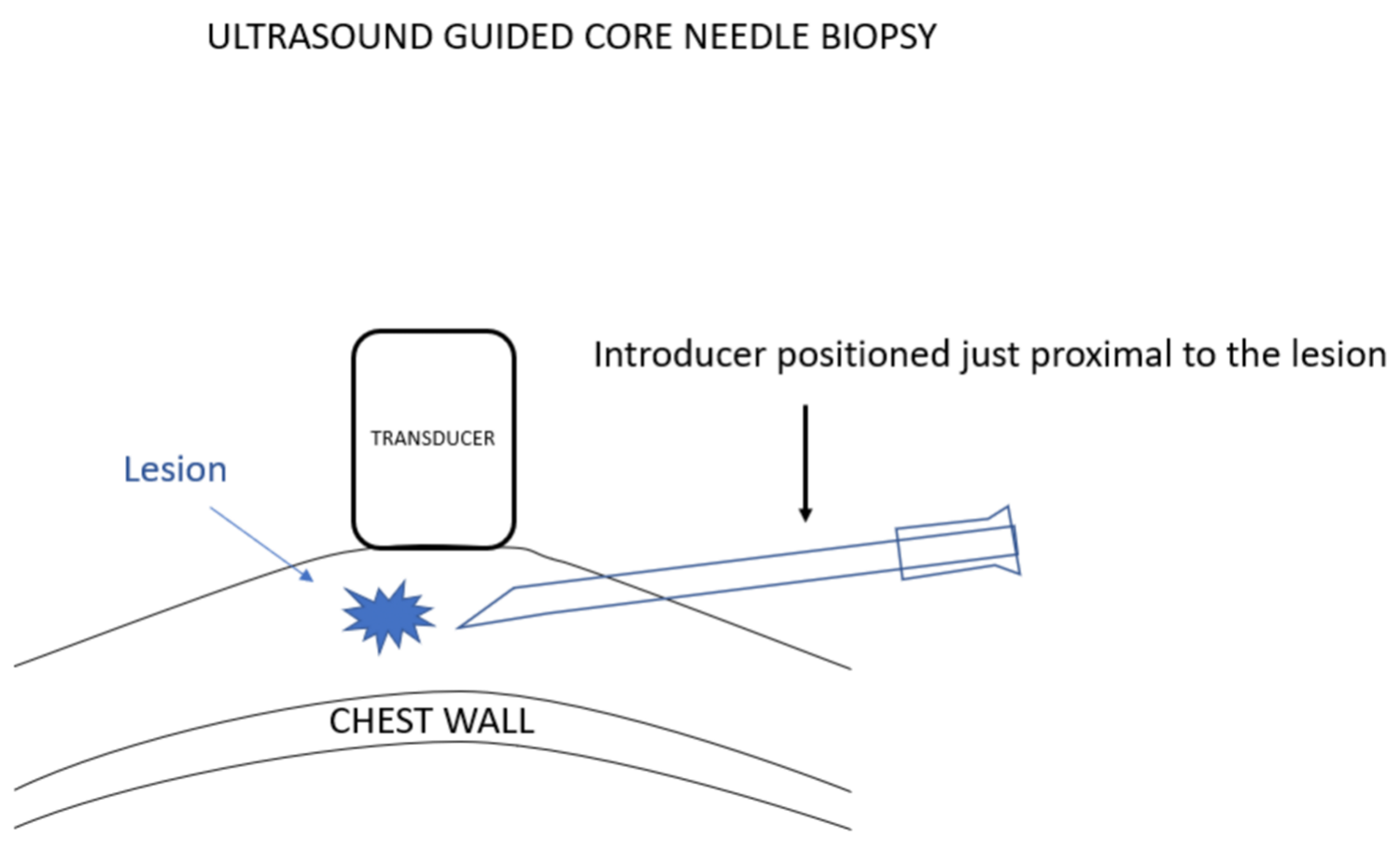
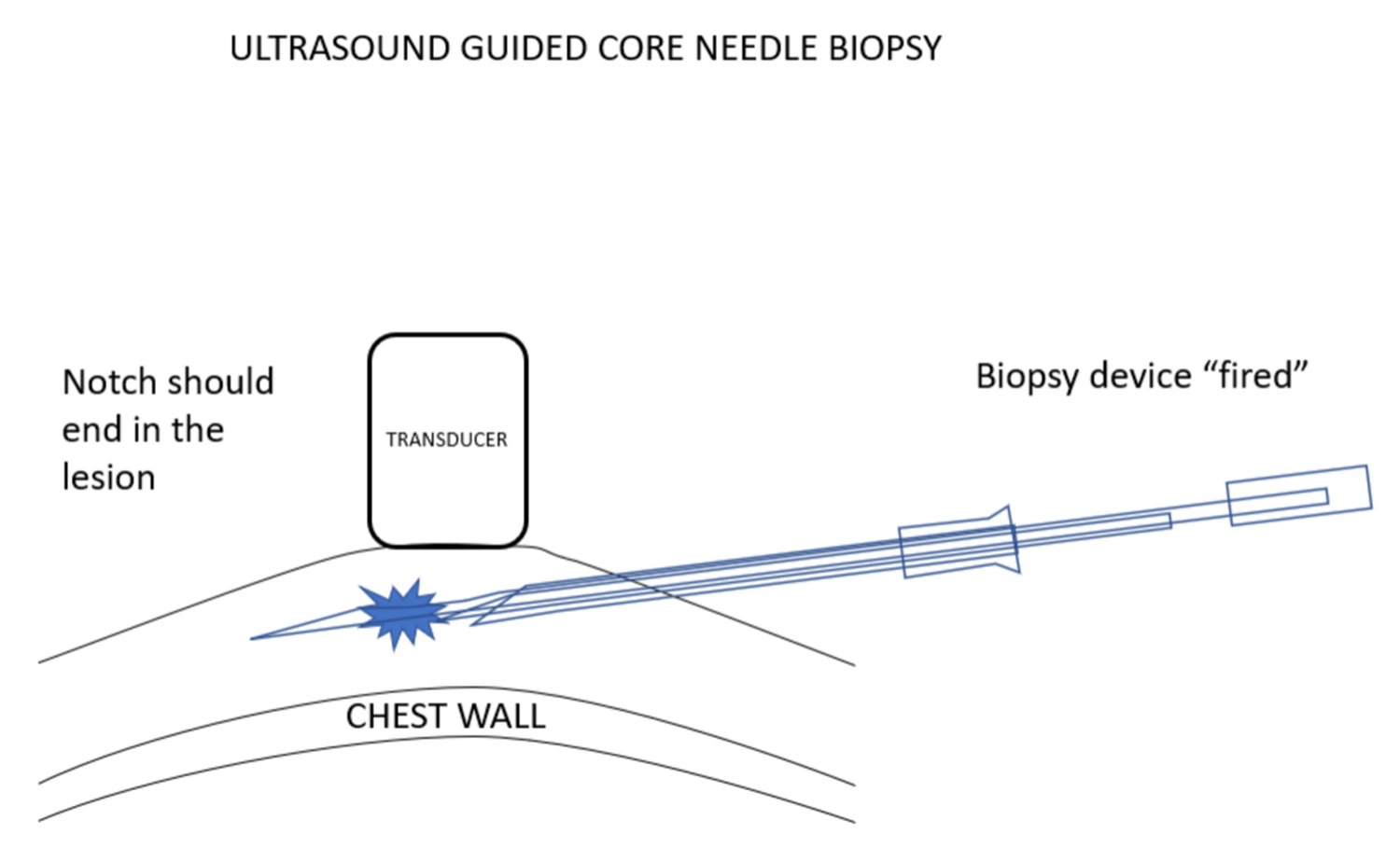
我更喜欢使用引导针,因为这样便于活检针多次插入,而不必多次有损伤性地穿过乳腺组织再返回病变处采样。在超声引导下置入引导针。超声探头的位置要使病变和针尖同时可见。选择最佳进针角度,以保证活检针穿过引导针并击发时,活检针尖将最终停在乳腺组织中,而没有穿刺到胸壁的风险。
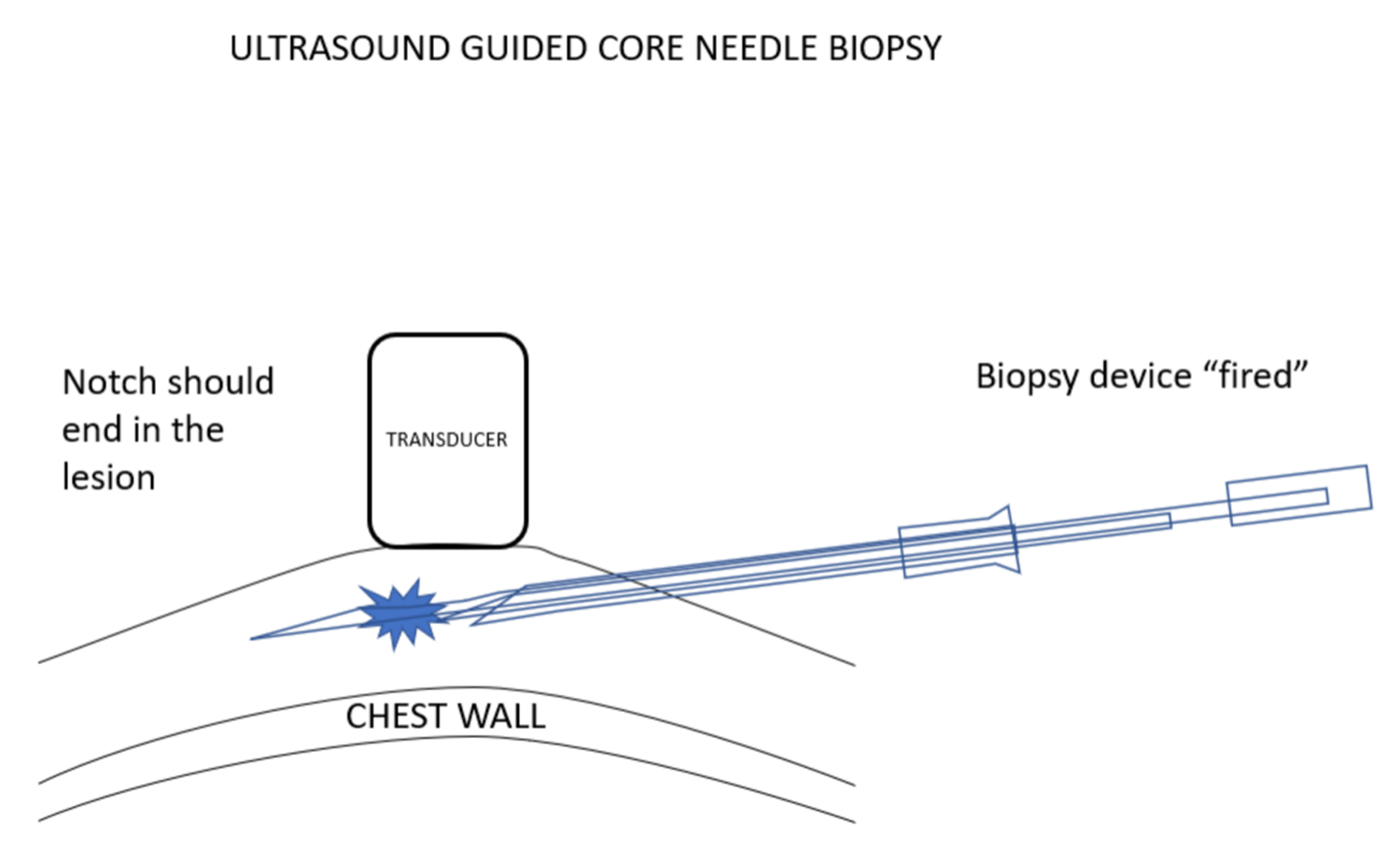
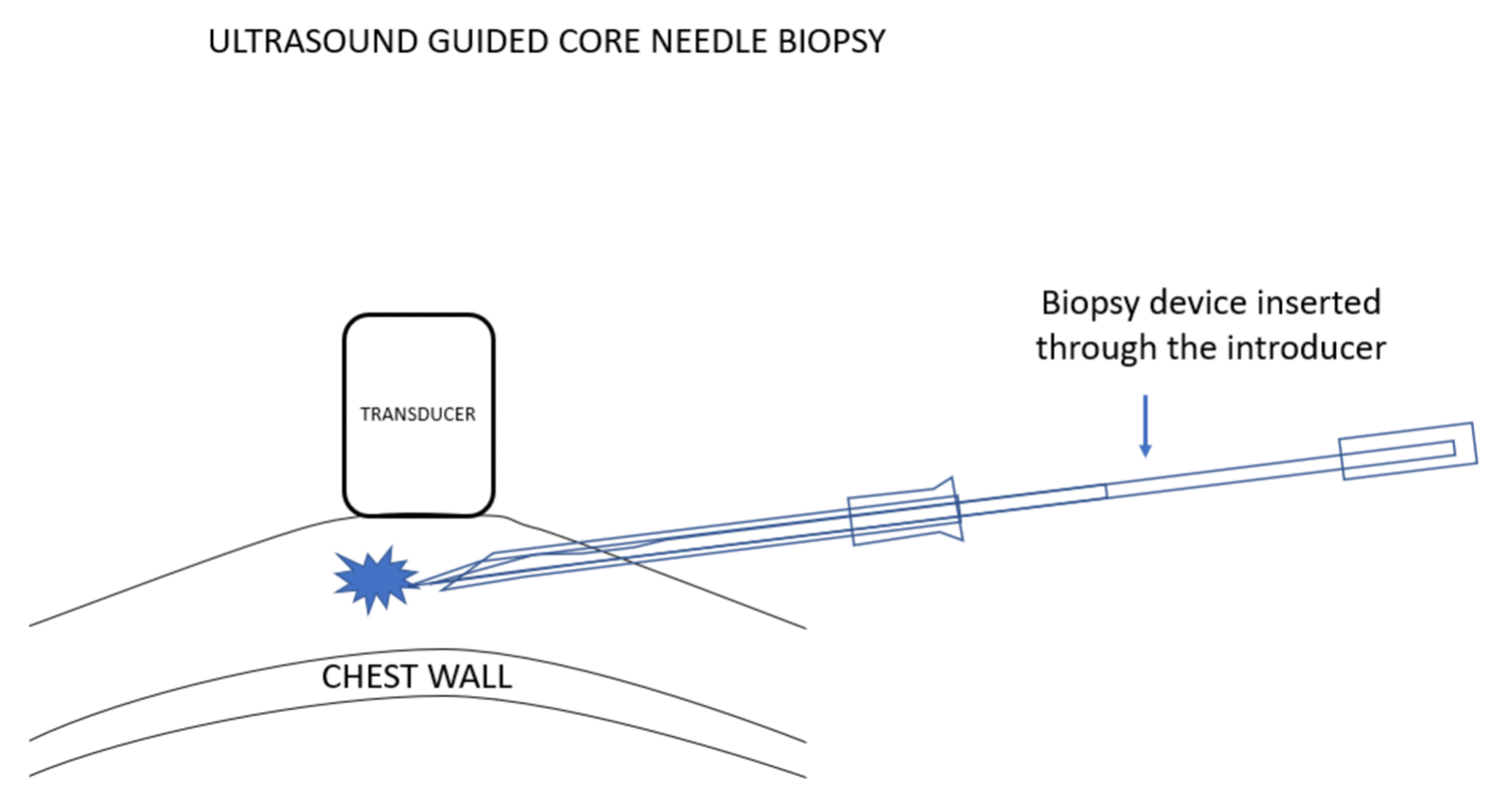
我更喜欢使用弹枪式活检针,因为常规的活检针进针时,由于病变经常被推开,通常很难将针尖插入乳腺病灶。组织的惯性与弹簧驱动活检设备的快速移动减少了这种情况的发生。使用这些设备时,必须确定,如果活检设备的尖端比预期移动得更远,它应该最终停留在乳房中,而不能到达胸壁或更远。
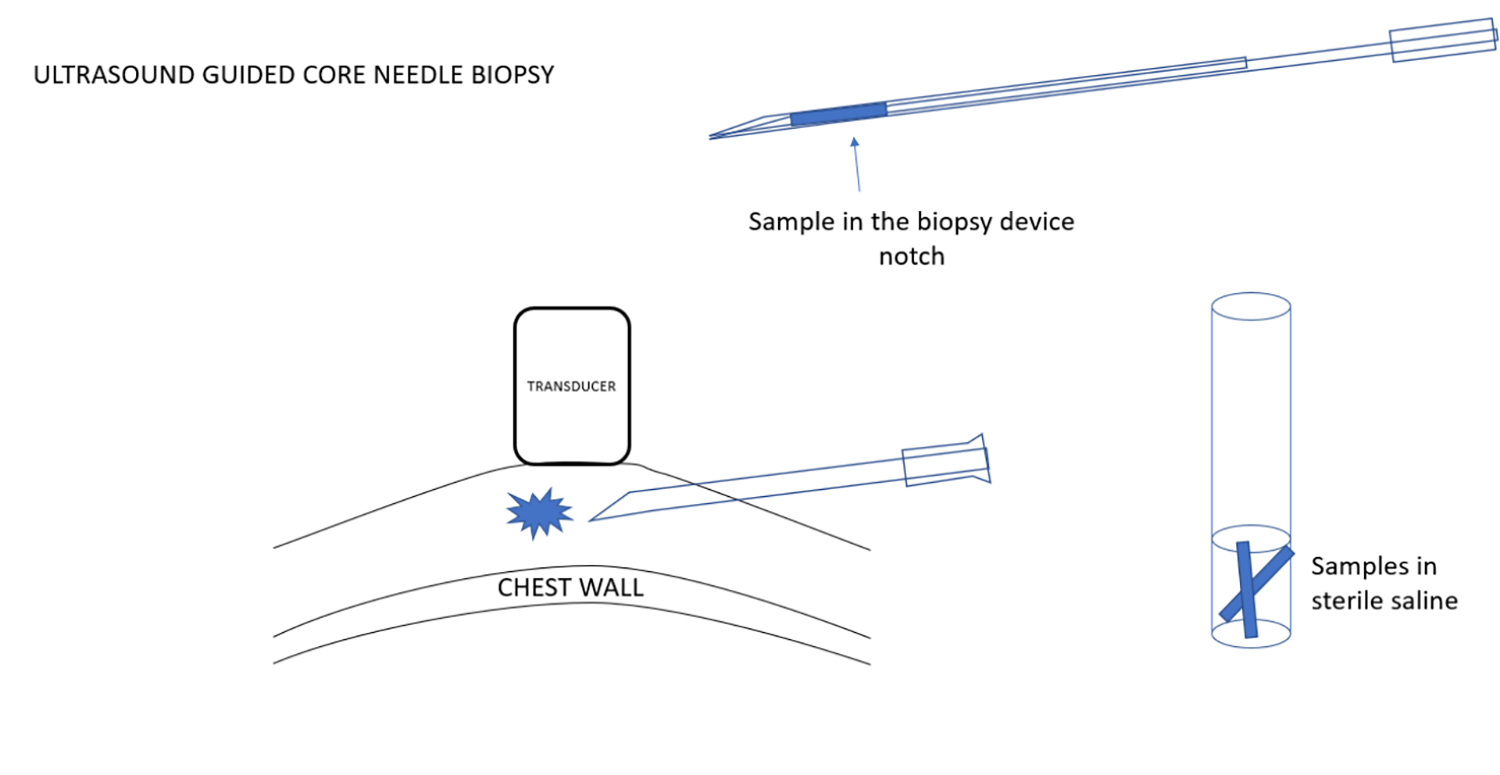
根据需要可一次取得多个标本。如果使用一次采一个标本的活检针系统,需要有一个无菌生理盐水的试管,可以插入针头和槽口,并清洗活检针的槽口,同时将组织样本移置到生理盐水中。因此,活检针可以保持无菌,不会接触任何防腐剂,安全地回到乳房中进行下一次取样。在手术结束时,可以将盐水管中保存的样本倒入大量的防腐剂中,以进行病理分析。
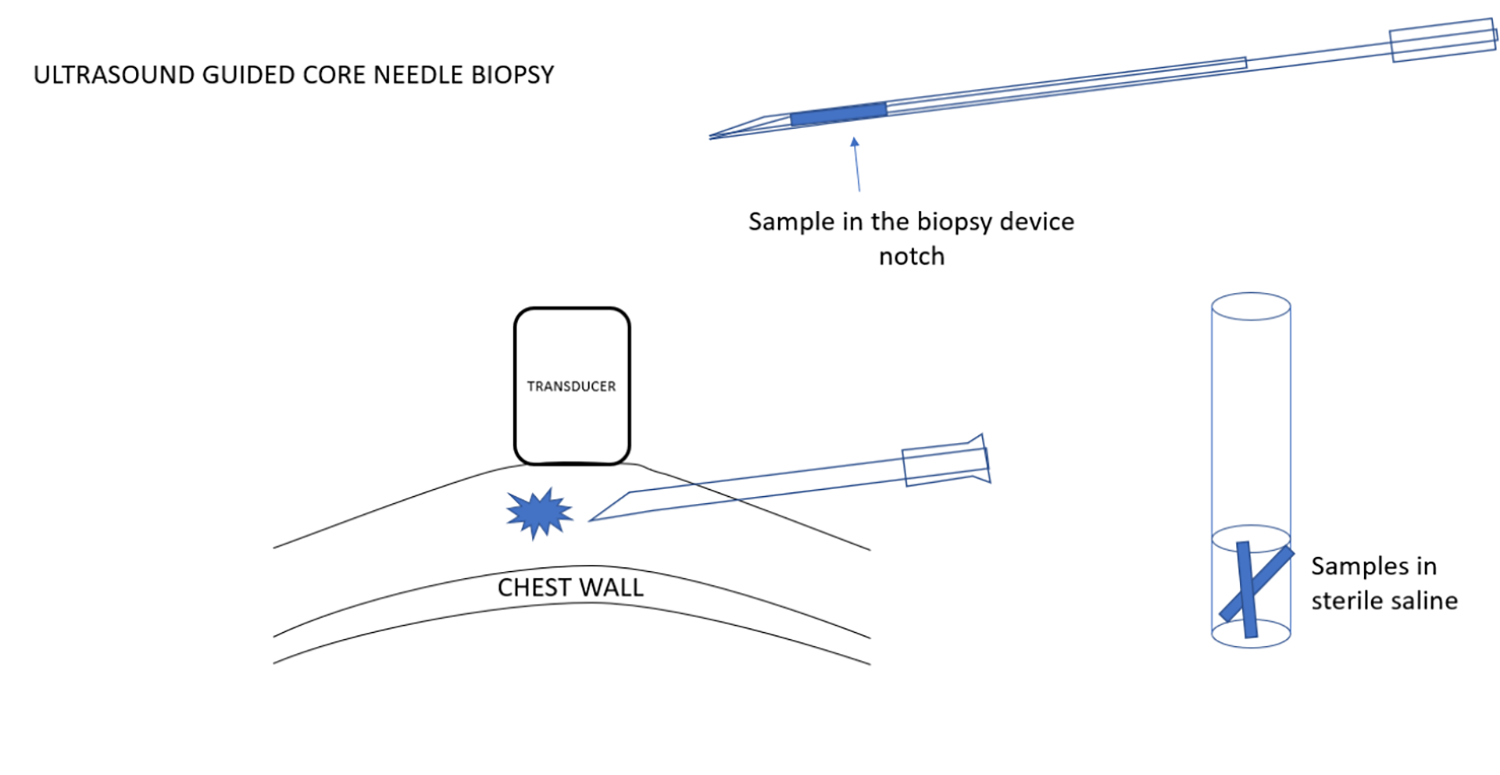
当手术完成后,抽出引导针,在活检和皮肤穿刺处加压进行止血。然后在皮肤穿刺点部位进行包扎。
与所有活检一样,应对病理结果进行评估,以确保其与影像学预期一致,并且采样完整。如果结果不一致,则应考虑进行进一步切除活检。
英文对照|ENGLISH COMPARISON
ULTRASOUND GUIDED CORE NEEDLE BIOPSY
SCREENING TO DIAGNOSIS
Mammography screening is one of the major advances in breast cancer care. Early detection is the main reason that the death rate from breast cancer has declined in the United States by over 40% since 1990. However, mammography screening is far from perfect. It does not detect all cancers and does not detect all cancers at a time when cure is possible. Furthermore, screening detects benign structures in the breast that are not cancer but cannot be differentiated from breast cancer based on the imaging. In the U.S. approximately 10% of women who participate in screening are recalled for additional evaluation with additional mammographic views or ultrasound. Many of these women can be told that the additional imaging shows that there is nothing of concern and they can return to annual screening. Others may be advised that there is no immediate concern, but that they should return in 6 months to be certain that what is seen on their mammogram is stable and likely is benign, while a small percentage of women (2% of the women screened) are advised to have an imaging guided needle biopsy to determine the histology of the lesion that was found on screening. I prefer core needle biopsy rather than fine needle aspiration (FNA) cytology because the histology provided by the cores provides a more complete diagnosis than cytological analysis.
ULTRASOUND IS THE BEST WAY TO TELL A CYST FROM A SOLID MASS
When there is an indeterminate lesion that is found by screening and confirmed by additional mammographic views (if needed), the next test is almost always ultrasound. Unless, using mammography, a circumscribed structure is found to contain calcifications that layer dependently and move with gravity indicating “milk of calcium”, mammography is unable to differentiate a simple, benign cyst from a solid mass.
Ultrasound can almost always confirm when a structure, that is indeterminate on mammography, is a cyst. Simple cysts are benign and need no further intervention unless they are causing pain. In that case, an image guided cyst aspiration may be useful, but this is very unusual. Image guided cyst aspiration can be performed using the same technique as for a biopsy, but, instead of inserting a biopsy device through an introducer under ultrasound guidance, an aspiration needle is used. The needle should be attached by connecting tubing to a syringe, and while the radiologist is inserting the needle under ultrasound observation, when the lesion is penetrated by the needle, an assistant can provide suction using the syringe.
ULTRASOUND GUIDED NEEDLE POSITIONING
Whether for guiding the aspiration of a cyst, or the biopsy of a solid mass, ultrasound is one of safest, easiest, and most accurate ways to guide the positioning of a needle into a lesion. The patient is positioned supine with the ipsilateral arm positioned behind her head to flatten the breast on the chest wall. The patient can be rolled to better position the breast. The goal is to position the patient, breast, and lesion to provide the safest access for the biopsy needle. Safety is of fundamental importance. Needles should always be positioned as parallel to the chest wall as possible so that when the sample is taken, the needle tip can pass beyond the lesion without reaching the chest wall.
The patient is scanned to determine the location of the lesion and the skin is marked directly over the lesion. Then, placing the ultrasound transducer over the lesion the location is marked for the skin entry point. The skin entry point is chosen so that when the needle enters the skin the needle tip can be seen at all times under the transducer as the needle is advanced toward the lesion.
The transducer is covered with a sterile sleeve that contains coupling gel. The needle entry location is then cleaned with betadine and alcohol and a sterile drape with an opening is placed with the skin entry point in the opening.
The skin entry point is infiltrated with local anesthesia.
I prefer using an introducing needle because this facilitates multiple insertions of the biopsy needle without having to make multiple damaging passes through the breast tissues to return to the lesion for sampling. The introducer is positioned under ultrasound guidance. The ultrasound transducer is positioned so that the lesion and the needle tip can be seen at the same time. The angle that the needle is introduced is chosen so that when a biopsy needle is passed through the introducer and “fired”, the biopsy needle tip will end in breast tissue with no risk of hitting the chest wall.
I prefer to use a spring-loaded biopsy needle because it is often difficult to push a needle into a breast lesion since the lesion often gets pushed out of the way. Tissue inertia with the spring driven rapid movement of the biopsy devise reduces the chance of this happening. Using these devices makes it important to be certain that if the tip of the biopsy device moves further than expected it will remain in the breast and will not reach the chest wall or beyond.
Multiple samples are obtained as needed. If a single sample system is used, I have a test tube with sterile saline that allows me to insert the needle and notch of the device and to wash the notch of the biopsy needle and remove the tissue sample into the saline. Thus, the biopsy needle is kept sterile and is never in contact with any preservative and can be safely passed back into the breast for the next sample. At the end of the procedure the samples that are held in the saline tube can be poured into a larger amount of preservative to be sent for pathological analysis.
When the procedure is complete, the introducing needle is withdrawn, and pressure placed over the biopsy and skin entry to provide hemostasis. Then a dressing can be applied to the skin entry site.
As with all biopsies, the pathology results should be evaluated to be certain that they are concordant with the imaging expectations and that the sampling was complete. If the results are not concordant, then an excisional biopsy should be considered.


