乳腺X射线摄影引导下的术前定位 (一)
概述
美国乳腺癌死亡率在1990年之前的50年一直都没有变化,但从1990年起这个数字就开始下降,究其原因就是从20世纪80年代中期起,美国开始通过筛查发现早期乳腺癌¹。随着我们不断提高发现早期乳腺癌的能力,死亡率还在持续下降;Tabar已经证实,如果癌症在1厘米或更小的时候被发现和治疗,患者10年生存率将超过90%²;因此,筛查需要具有积极诊断早期乳腺癌的能力。
使癌症早期发现变得更加复杂的原因是,有许多通过筛查发现的良性病变(囊肿、纤维腺瘤等),如果不进行组织学或病理学分析,是很难与恶性病变进行鉴别诊断的。在美国,通过乳腺X射线摄影引导的空芯针活检的病灶中,乳腺癌比例为20%-40%。通过影像引导下的空芯针活检获得组织病理结果来确诊筛查发现的病变这种方式已经成为临床标准。活检可以在X射线,超声,MR,DBT,或对比增强X射线检查引导下进行。
最终,如果是通过手术活检的方式来确定组织病理结果,或者在通过空芯针活检获得的组织病理结果不明确或提示恶性,则手术切除仍然是主要干预治疗手段。还有当诊断为癌症后,需要通过手术切除病变。我们需要帮助外科医生确定病灶位置,以便他们能够安全、准确地切除需要切除的部分,同时尽可能保留更多的正常乳腺组织。
发展历史
在筛查的早期,外科医生通过一段时间才接受了这样一个事实:乳腺X射线摄影发现的早期乳腺癌非常小,即便知道病灶位置,也无法触诊到。在影像引导定位技术发明之前的一段时期内,外科医生会根据他们对病灶位置的大致估计,切除大量的乳腺组织。很显然,当一位经验丰富的外科医生都不能在病人已经切除的外上象限组织中找到仅在乳腺X射线摄影上发现的癌症病灶时,我们放射科医生应该帮助外科医生去确定病灶的位置。我们还应该认识到,那些需要引起重视或活检的病灶中,有20%-40%最终被证实是恶性的(60%-80%不是乳腺癌);外科医生需要安全、精准的引导来准确切除病灶,最大程度的减少乳房损伤并保持外观的完整性;起初,活检需要在手术室全麻下进行,成本昂贵,而且使病人面临不必要的风险。我们意识到,如果我们放射科医生能够准确地指导外科医生,那么该手术就可以在门诊手术室局麻下进行,更安全,也更高效。
早期的乳腺穿刺定位
早期的穿刺定位术,是使用普通皮下注射针进行的,根据乳腺X射线摄影图像将其放在可疑病灶附近。这是有问题的,因为针是徒手放置并且不是总能靠近病灶;另外,在定位后,患者被安置于外科手术体位或对乳房进行术前无菌消毒时,这些被放置好的注射针可能会随着乳房的运动而脱落。最终,我发明了一种导丝,只要针放置准确,金属钩丝就通过针鞘向前推进,钩丝末端弯曲的方式使其可以弹开并钩住腺体组织³。手术前,我们可以在影像引导下放置钩丝并将其固定,外科医生可以沿着钩丝找到需要切除的病灶组织。我还开发了导丝定位术,安全且非常准确⁴′⁵。
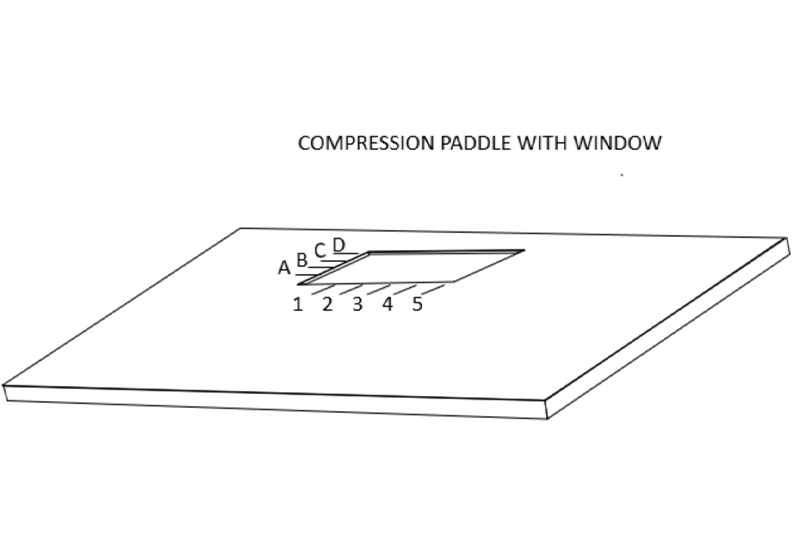
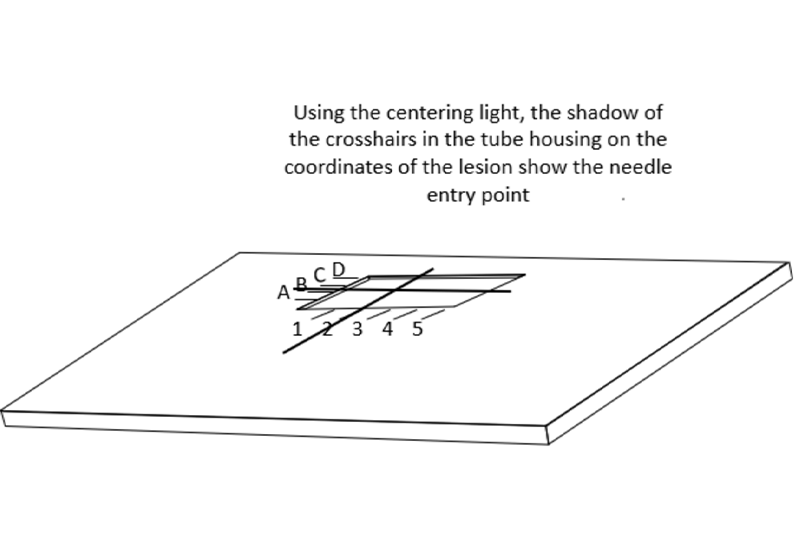
乳腺X射线摄影引导下的精准定位需要一块带有活检窗的压迫板。在乳腺X射线摄影引导下的定位术时,需要用带活检窗并且窗口边缘有标记的压迫板压迫乳房。当乳房定位,病灶在乳房X射线影像的活检窗内,可以使放射科医生确定病灶的X坐标和Y坐标。
校准定位灯中心光束
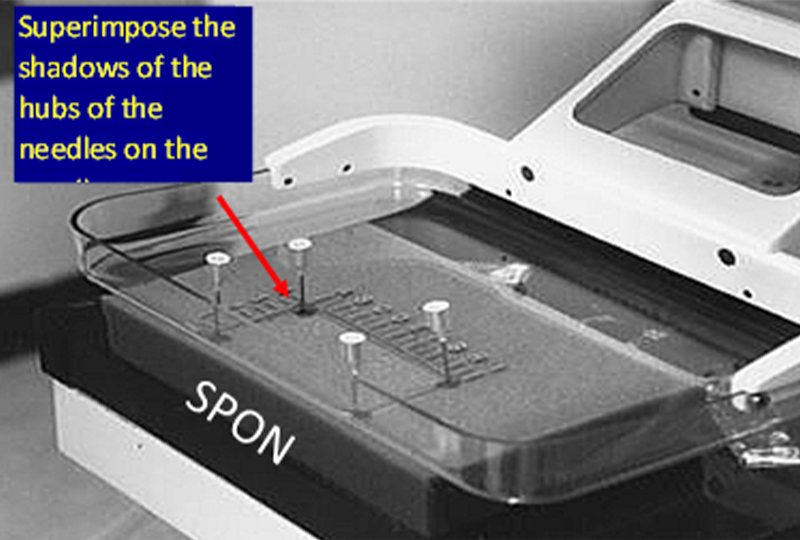
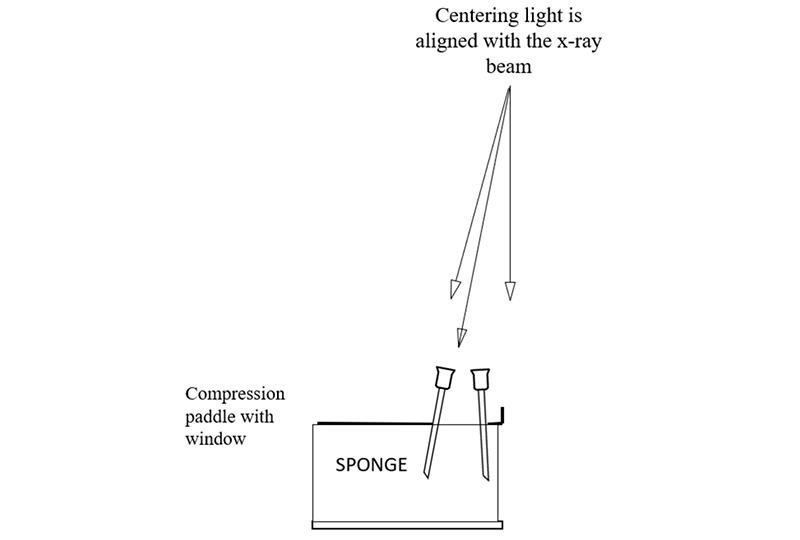
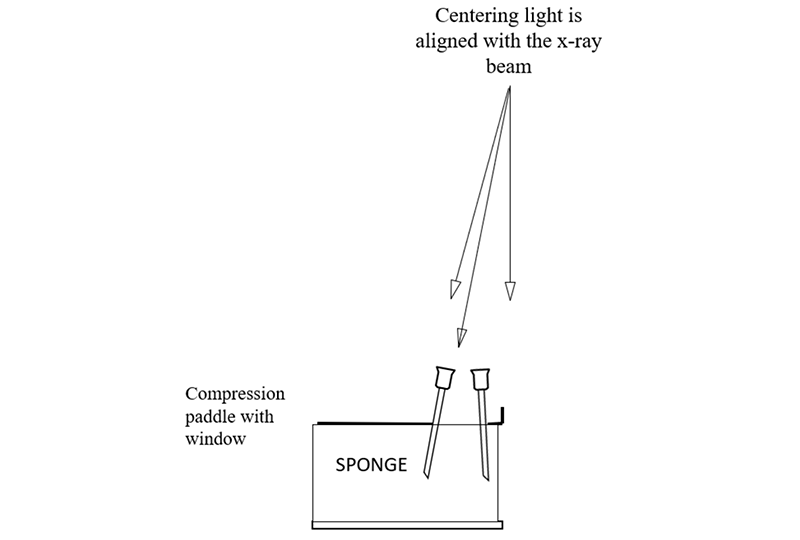
为了使定位针在乳房中准确定位,乳腺机机架上的定位灯中心要与X射线束对齐。可以通过在带有活检窗口的活检压迫板下放置一块海绵来检查,压迫到位后,将四根针分别放置在活检窗的四个边缘角。
打开定位灯,将每根针定位,使针头的阴影位于已穿过海绵的针阴影的中心,然后将针尖继续向下推进海绵。操作正确的话,针看起来是斜的,而不是垂直于压迫板或者探测器;因为跟X射线束一样,定位灯的光束也是发散的,它从压迫板和探测器胸壁侧中心向外倾斜(靠胸壁侧中心几乎垂直)。
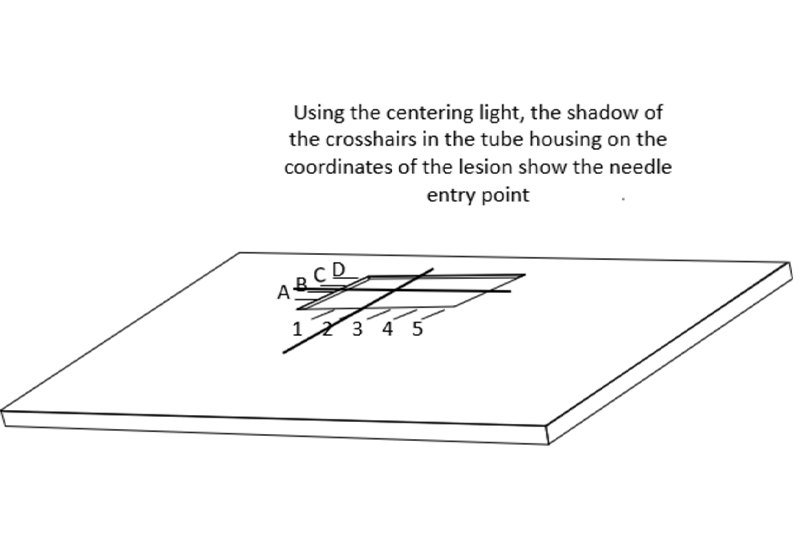
当针对齐并且拍摄X线图像后,如果图像中显示针头投影与针轴不在一个中心上,说明X射线与定位灯中心光束没有对齐,这时就需要纠正。只要中心光束与X射线束对齐,就可以像透视镜一样通过定位针沿着病灶方向进行准确的定位。
定位患者
当发现病灶并确定需要手术时,我们需要确定病灶在乳房中的三维位置;放射科医生应该选择病灶离皮肤最近的穿刺路径,进针时,最重要的是针需要与胸壁保持平行,不然会不小心穿到胸壁甚至肺部或者纵隔。
当确定了最短进针路径后,要保证针与钩丝的足够长度,我一般会选择比预估皮肤到病灶的进针距离更长的钩丝针。这点很重要,因为在手术过程中,要确保钩丝针尽可能长,使之可以穿过病灶,使钩尖刚好在病变外的位置,病灶处于钩丝加粗部分。
然后选择最佳的投照位置进行患者摆位,不是一定要常规位置,将病灶区域的皮肤放置于活检窗内,因为我们的目的是用最短的进针路径定位病灶,外科医生沿着钩丝确定病灶位置,减少不必要的切除。
病人应该尽可能舒适地坐着。我遇到过的唯一重大问题是患者出现血管迷走神经反应。手术团队应该为此做好准备。理想的情况是,如果病人出现昏厥,椅子可以倾斜到特伦德伦伯卧位(Trendelenburg position)。手术过程中,操作者最好可以与患者进行一些无关话题的聊天,分散其注意力,重要的是不要让患者看到针,因为这可能会导致病人出现血管迷走神经反应。一般来说,在针穿刺进病人乳房之前,我会转动病人的头让她看不到针,同时打趣的说到:“你有一张可爱的脸,但我需要把它从影像上移开”。在整个手术过程中,患者的乳房需要保持不动,但操作者应尽努力去分散患者注意力,减少其担忧。
中英翻译
INTRODUCTION
Screening and the earlier detection of breast cancer, that began in the mid 1980’s in the U.S., is the main reason that deaths from breast cancer, unchanged for 50 years, began to fall in 1990¹. The death rate has continued to decline as we have improved our ability to detect breast cancers earlier. Tabar has shown that if cancers are detected and treated when 1 cm or smaller, the 10 year survival is over 90%². Consequently, screening requires an ability to aggressively diagnose small cancers.
Compounding the problem of early detection is the fact that there are many benign lesions (cysts, fibroadenomas, and others) that are detected by screening that cannot be differentiated from malignant lesions without some form of histological or pathological analysis. The yield of cancers on image guided needle biopsies of mammographically detected lesions in the U.S. is 20%-40%. In the U.S. image guided core needle biopsy, that provides histological information, has become the standard of care to determine the importance of a screen detected lesion. These needle biopsies can be guided by Full Field Digital Mammography, Ultrasound, Digital Breast Tomosynthesis, Magnetic Resonance Imaging and Spectral Mammography.
Ultimately, if surgical biopsy is the main method for obtaining histological diagnosis, or if the core needle biopsy result is indeterminate, or suggestive of malignancy, surgical excision remains the main intervention. This is also the case when the diagnosis of cancer has been made and the lesion needs to be surgically removed. Consequently, we need to be able to guide surgeons to areas of concern so that they can safely and accurately remove what needs to be removed while not sacrificing breast tissue, unnecessarily.
HISTORY
In the early years of screening, it took a while for surgeons to accept the fact that mammography was finding breast cancers that were so small that they could not be palpated even when the location of the lesion was known. Prior to the development of image directed guide placement, there was a period when surgeons would remove large amounts of breast tissue based on their estimate of the expected location of a lesion. In my own experience, when a senior surgeon removed the upper out quadrant of a patient’s breast and missed what ultimately proved to be a cancer that was only visible on mammography, it became clear that we needed to guide surgeons to the area of concern. We also recognized that since 20%-40% of the lesions that raised concern and needed to be biopsied, proved to be malignant (60%-80% are not cancer), the surgeon needed safe and accurate guidance to accurately remove a lesion while minimizing any cosmetic damage to the breast. In the beginning these biopsies were performed under general anesthesia in the main operating rooms. Since this was expensive and placed patients at unnecessary risk, we realized that, if we could accurately guide surgeons, the procedure could be done in an outpatient operating room using local anesthesia with much greater safety and efficiency.
EARLY “NEEDLE LOCALIZATIONS”
The early “localizations” were performed using plain hypodermic needles that were placed close to the suspicious lesion based on the mammogram. These were problematic since the needles were placed “free hand” and were not always close to the lesion. In addition, they could fall out with the motion of the breast when the patient was positioned for surgery or when the breast was being prepared with sterile solutions prior to the operation. Ultimately, I invented a wire guide that, once the needle was accurately placed, the guide could be passed through the needle. The end of the wire was bent in such a way that allowed it to spring open and hook into the tissue³. The wire can be placed under imaging guidance prior to surgery and the guide positioned so that the surgeon can follow the wire to the volume of tissue that needs to be removed. I also developed techniques for positioning the wire guide that were safe and very accurate⁴′⁵.
Accurate mammographically guided needle positioning requires a fenestrated compression paddle.
For mammographically guided needle localization the breast is held in compression using a paddle that has a window in it with markings along the edges of the window.
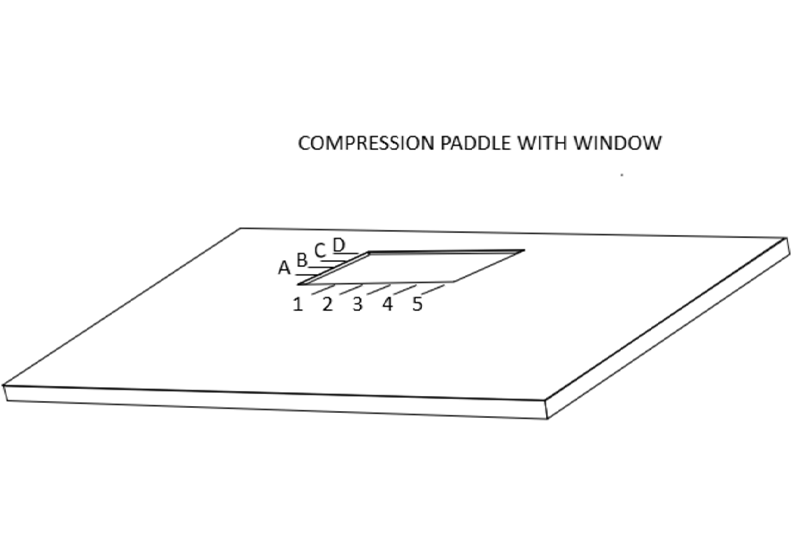
This allows the radiologist to determine the x and y coordinates of the lesion when the breast is positioned so that the lesion is visible on the mammogram “in the window”.
ALIGNING THE CENTERING LIGHT
In order to facilitate the accurate positioning of needles in the breast, the centering light in the mammography gantry needs to be aligned with the x-ray beam. This can be checked by placing a sponge in the compression system with the window compression in place so that needles can be placed in the corners of the window.
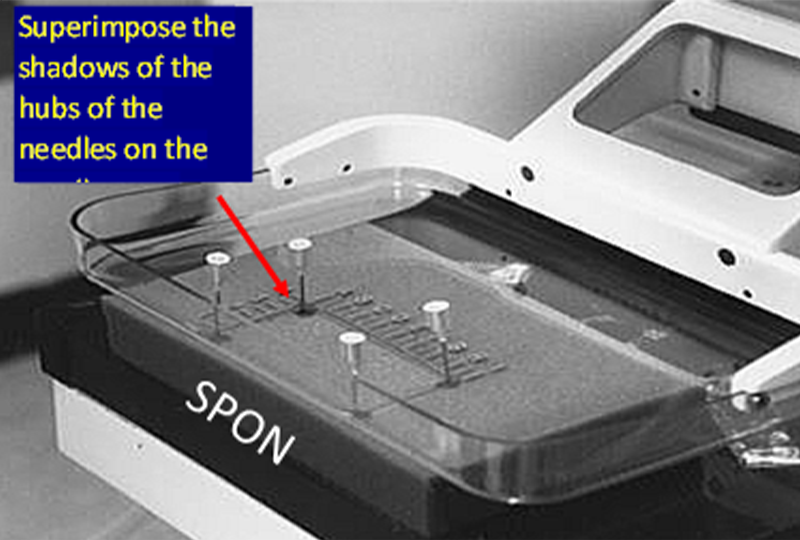
The centering light is turned on and each needle is positioned so that the shadow of the hub of the needle is centered on the shadow of the needle where is penetrates the sponge, and then the needle is advanced into the sponge. If done correctly you will see that the needles are tilted and not perpendicular to the paddle or detector. This is because, like the x-ray beam, the centering light is divergent, it angles out from the center of the chest wall side of the paddle and detector (where it is almost perpendicular).
When the needles are aligned, an x-ray image is then taken. If the shadows of the needle hubs are not centered on the needle shaft in the images, then this means that the centering light is out of alignment and this needs to be corrected. Once the centering light is aligned with the x-ray beam then it can be used like a fluoroscope to position the localization needle accurately through or alongside the targeted lesion.
POSITIONING THE PATIENT
Once a lesion has been identified and the decision is made to perform a surgical excision, the three-dimensional location of the lesion in the breast needs to be determined. The radiologist then should pick the shortest distance to the lesion from the skin when the needle is passed parallel to the chest wall. This latter requirement is important so that it is virtually impossible to, inadvertently, puncture the chest wall, or worse into the lung or the mediastinum.
When the shortest distance has been determined, I select a needle wire combination that is longer than the distance that I am expecting from the skin to the lesion. It is critical to be certain that the needle and wire are “too long” so that they can pass beyond the lesion during the procedure to be certain that the hook is just beyond the lesion with the lesion on the thickened segment of the wire guide.
The patient is then positioned in the best mammographic position to place the skin entry site in the window of the compression paddle. There is no need to adhere to specific mammographic projections since the goal is to place the guide in or alongside the lesion using the shortest distance to the lesion so that the surgeon, following the wire to the lesion does not have a long dissection.
The patient should be seated and as comfortable as possible. The only major problem I have ever had is when a patient has a vasovagal reaction. The team should be prepared for this. Ideally the chair can be tilted into the Trendelenburg position should the patient become faint. It is best if the technologist can distract the patient during the procedure with unrelated talk. It is important to not allow the patient to see the needle since this can result in a vasovagal episode. I turn the patient’s head so that she cannot see the needle before I introduce it into her breast while jokingly stating “now you have a lovely face, but I need to move it out of my pictures”. The patient’s breast needs to remain stationary throughout the procedure, but every effort should be made to distract her and not raise her concern.
REFERENCES
1.Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019 May 1;125(9):1482-1488.
2.Tabàr L, Fagerberg G, Duffy SW, Day NE, Gad A, Gröntoft O. Update of the Swedish two-county program of mammographic screening for breast cancer. Radiol Clin North Am. 1992 Jan;30(1):187-210. PMID: 1732926.
3.Kopans DB, Deluca S. A modified needle-hookwire technique to simplify the preoperative localization of occult breast lesions. Radiology 1980; 134:781.
4.Kopans DB, & Meyer JE, The Versatile Spring-Hookwire Breast Lesion Localizer. AJR 1982;138: 586-587
5.Kopans DB, Meyer JE, Lindfors KK, & McCarthy KA, Spring-Hookwire Breast Lesion Localizer: Use with Rigid Compression Mammographic Systems. Rad 1985;157: 505-507


